 |
 |
 |
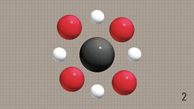
Methane is a simple
hydrocarbon made up of four hydrogen
atoms and one carbon atom.
|
Oxygen can easily reach
every part of a simple hydrocarbon like
methane.
|
For this reason, methane
burns almost completely, leaving little
or no pollution.
|
 |
 |
 |
|
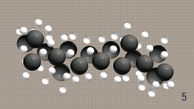
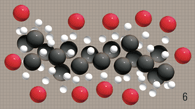
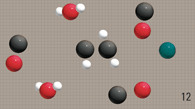
Gasoline and diesel hydro-carbons
tend to form a complex structure. |
Unfortunately these
hydro-carbons tend to get bundled up with
one another, creating clusters.
|
When hydrocarbons bundle,
oxygen cannot
reach all the fuel.
|
 |
 |
 |
Therefore, the
hydrocarbons in gasoline, diesel,
fuel oil, etc., do not burn
completely.
|
The result of this
incomplete burn is exhaust. These
partially burnt molecules contribute to
pollution.
|
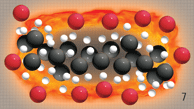
With the addition of
Green Plus, these molecules are
catalyzed, allowing the hydrocarbons to
unbundle.
|
 |
 |
 |
During this unbundling,
these hydrocarbons are
more readily
exposed to oxygen molecules,
creating a reaction.
|
This reaction is a more
complete burn of the hydrocarbon chains.
|
The result of a more
complete fuel burn is a reduction of
emissions and increased fuel efficiency.
|
